On thermodynamics and evolution
 Often in my perusing of the internet, generally on Twitter, I come across some creationist espousing that the second law of thermodynamics as proof that evolution canít possibly happen. Now anyone with a grasp of thermodynamics knows that this ďargumentĒ is a bunch of bovine scatology, but on Twitter it is hard to combat it more than with ďthe Earth isnít a closed systemĒ. With this in mind this post on what the laws of thermodynamics actually say and what they mean. Feel free to link here if you get the thermodynamics argument from a creationist.
Often in my perusing of the internet, generally on Twitter, I come across some creationist espousing that the second law of thermodynamics as proof that evolution canít possibly happen. Now anyone with a grasp of thermodynamics knows that this ďargumentĒ is a bunch of bovine scatology, but on Twitter it is hard to combat it more than with ďthe Earth isnít a closed systemĒ. With this in mind this post on what the laws of thermodynamics actually say and what they mean. Feel free to link here if you get the thermodynamics argument from a creationist. To start, thermodynamics is the study of how heat flows in systems. It is immensely useful when designing pretty much anything that has to move as it deals with the limitations of how heat and work interact. It is also useful in physics in general as it can be to some extent expanded to energy flow in general. How this all works out will become clearer as we go through the various aspects of thermodynamics.
Now in general thermodynamics can be seen as a set of three-ish laws that in general terms set out how heat can flow in and out of systems. We will look at each of these in turn and see how they affect the ability of order to come from chaos.
The first law we will look at is actually called the Zeroth Law of Thermodynamics. Some definitions:
If bodies A and B are each in thermal equilibrium with a third body T, they are in thermal equilibrium with each other. (Halliday & Resnick 1988, p448)
The zeroth law of thermodynamics states that when two bodies have equality of temperature with a third body, then in turn they have equality of temperature with each other. (Van Wylen & Sonntag 1973, p33)
So what does this mean? Simply put if two systems are in thermal equilibrium (that is, the same temperature as) a third system, then the two original systems are in thermal equilibrium with each other. In other words if system A is touching system B and system C is touching system B but system A and C are not touching each other; and further if A and B are the same temperature and B and C are the same temperature then A and C are also the same temperature. The implication of this will become more apparent as we look at the second law.
The next law, the First Law of Thermodynamics (second in order) deals with the creation and destruction of heat:
during any cycle a system undergoes, the cyclic integral of the heat is proportional to the cyclic integral of the work. (Van Wylen & Sonntag 1973, p 90)
The quantity Q-W is the same for all processes. It depends only on the initial and final states and it does not matter at all how you get from one to the other. (Halliday & Resnick, 1988 p469)
Mathematically the first law is stated thus:
![]()
Where delta U is the change in the internal energy of the system, Q is the heat in the system and W is the work done by the system.
This is basically the law of conservation of energy, that is the amount of energy in an isolated system is constant. If the heat of the system increases, the work done by the system decreases and vice versa. In simpler terms you canít get more work out of a system than energy you put into the system. This law puts the ultimate limit on fuel economy for example. There is only so much energy in a litre of gasoline and even if the car is 100% efficient at converting that energy in to movement, the car will still only get a finite amount of kilometres per litre of fuel.
Now the Second Law of Thermodynamics can be broken down into a few parts. Firstly:
The second law of thermodynamics, statement A: It is impossible to extract heat from a system and convert it wholly into work with out causing other changes in the universe. (Radin & Folk 1982 p 336)
Second law - first form: It is not possible to change heat completely into work, with no other change taking place. (Halliday & Resnick 1988, p510)
Basically the first part of the second law says that you canít convert energy into work with 100% efficiency. That is there will be some loss of the energy to the environment through some mechanism, usually friction. This is the part of thermodynamics that prevents the creation of perpetual motion machines for example. There is always something that is going to steal some of your energy away, be it friction or gravity. Itís all a matter of degree.
In mathematical terms the first part of the second law looks like this:
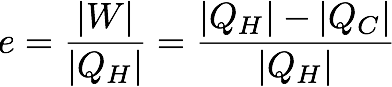
In this case e is the efficiency of the heat engine in percent. W is the useful work out of the engine which is also equal to the total heat used by the system (Q_H) minus the heat lost to the environment (Q_C). The work is divided by the total heat used by the system (Q_H) to get the efficiency.
Now the second part of the second law of thermodynamics goes like this:
The second law of thermodynamics, statement B: Heat can never, of itself, flow from a lower to a higher temperature. (Radin & Folk 1982, p 337)
Second law - second form: It is not possible for heat to flow from one body to another body at a higher temperature, with no other change taking place. (Halliday & Resnick, 1988, p512)
So what does this mean? Quite simply it means that heat always moves from hot objects to cool objects and not the other way around. That is a cold object will suck heat out of a hot object until they are in thermal equilibrium (i.e. at the same temperature). Of course it is possible to move heat from a cold object to a warm object, our refrigerators do it all the time, but doing so requires energy from an outside source.
This brings us to the last part of the Second Law:
The second law of thermodynamics statement c: (i) The integral of ![]() is the same for all reversible processes between the same states. (ii) The total entropy change in any given process is positive or zero. (Radin & Folk 1982, p 345)
is the same for all reversible processes between the same states. (ii) The total entropy change in any given process is positive or zero. (Radin & Folk 1982, p 345)
isolated systems tend toward disorder and entropy is a measure of this disorder. (Serway & Faughn, 1986)
What this means is that the random disorder in a system, that is the heat of a system, must increase or stay the same over time. The key here is that the system be in isolation. If the system isnít isolated (or closed) then the disorder can be reduced (as is the case in a refrigerator) by the application of energy to transfer the entropy somewhere else.
So how does this relate to creationist ďargumentĒ that the Second Law of Thermodynamics prevents evolution as greater order goes against entropy? Well as far as I can tell the ďargumentĒ goes something like this:
- The Second Law of Thermodynamics says entropy has to increase
- Since entropy is disorder, disorder must therefore increase
- Evolution says complexity came from simplicity
- Since disorder has to increase, complexity canít come from simplicity therefore some form of god has to intervene
Now let us look at this step-by-step. First looking at the Second Law of thermodynamics argument. The statement that the Second Law says that entropy must increase is only partially true. As Iíve shown above entropy only tends to increase and that the application of energy can cause entropy to shift around. That is entropy can be made to move from an area of higher entropy to an area of lower entropy by applying energy. Again as Iíve provided above, a simple example is the lowly refrigerator. In the refrigerator the items inside are made cooler than the surrounding environment. Taking the creationist argument about entropy this is impossible. Which it is if there wasnít energy being input into the system that we call a refrigerator. This energy allows the refrigerator to pump heat (and thus entropy) against the gradient, that is from cold to hot, or from disorder to order. So by stating that entropy has to increase, the creationist is only half telling the truth. As long as thereís energy available, entropy can be moved from place to place or at least be held at bay.
This effectively destroys the first and second premises of the creationist argument. The Earth isnít a closed system. There is continual energy input from the Sun. A lot of energy input Ė to the tune of about 1.7e17 J of energy per second over the whole lit surface of the Earth. Thatís about 170000 Terawatts of power. Thatís ample energy to create order from disorder.
At this point the craftier creationists will then claim that the universe is a closed system so that their half-interpretation of the Second Law still holds. Well yes, except that thereís those pesky stars pumping energy into the system again, and gravity giving everything gravitational potential energy. The reality is that as long as there is something pumping energy into the universe, there will be the ability to hold the line on the amount of entropy in the universe. Also missing from their argument is that the universe is incredibly big. It can absorb a heck of a lot of entropy before things start falling apart. The reality is that the universe wonít undergo itís ultimate heat death, that is the universe moving to thermal equilibrium until the last of the black holes evaporates which is on the order of a googol years from now.
At this point some creationist wiz will break out the First Law (in itís guise as the conservation of energy) and state that energy canít be created or destroyed. Which is true but these same creationists for some reason forget that matter is also a form of energy. Stars get their vast amounts of energy from converting a little be of matter from each nuclear fusion reaction into energy. So the star gets a little lighter and pumps out some more entropy defeating energy. In practical terms the stars are taking the entropy around them and transferring it to their cores. In the end the stars run out of convertible matter and they die, releasing heavy elements to the cosmos where gravity will pull it all together again to form another star and possibly some planets.
So where does this leave the creationist argument? Well premise 3 depends on the veracity of premises 1 and 2, and since premises 1 and 2 are complete nonsense if you look at the whole of the laws of thermodynamics (not just the bit that you think are the laws of thermodynamics) premise 3 also collapses. This leaves the conclusion at 4 with nothing to stand on.
To sum all this up, if you come across someone using the laws of thermodynamics to refute a perpetual motion machine, then youíve probably come across someone with some grounding in physics and the laws of thermodynamics. On the other hand if you come across someone who thinks the laws of thermodynamics would prevent evolution from happening, then youíve come across someone who has no idea whatsoever about physics and thermodynamics.
References
Halliday, D. and Resnick, R., 1988, Fundamentals of Physics, 3rd ed., New York, Wiley
Radin, S.H. and Folk, R.T., 1982, Physics for Scientists and Engineers, Englewood Cliffs, Prentice-Hall
Serway, R.A. and Faughn, J., 1985, College Physics, Philadelphia, Saunders
Van Wylen, G.J. and Sonntag, R.E., 1973, Fundamentals of Classical Thermodynamics, New York, Wiley
